Looking back at FDA product recall data from 2012 to 2024
It seems like every day there’s a headline for a new recall. From food products to medication and medical devices, these incidents impact a wide range of industries, businesses, and consumers. Between 2012 and 2024, the FDA recalled a total of 94,288 products. We analyzed the product recall data to understand the source and impact of these incidents. Read this report to learn out top insights!
Overview of FDA product recall data
The U.S. government passed the 1906 Pure Food and Drugs Act to stop the distribution of “adulterated and misbranded food and drugs.” The Food and Drug Administration formed under this law and got its name in 1930. Today, it’s the oldest consumer protection agency in the country.
One of the major functions of the FDA is regulating consumer products, so they’re safe, effective, and secure. Companies can issue voluntary recalls for their products, but sometimes the FDA requires manufacturers to remove products from the market. The FDA recall dashboard includes data on both types of events.
Types of products recalled
The most common product that the FDA recalls is medical devices. This category includes things like suture kits, lasers, pumps, surgical instruments, and more. The other recall categories are food and cosmetics, drugs, biologics, veterinary, and tobacco.
Total recalls by product type


These figures align with the commonality of each category. For example, the majority (roughly 80%) of U.S. adults don’t use tobacco products. So, it makes sense that the FDA recalls far fewer tobacco products each year than food products.
Recalling firms
The FDA collects data on recalling firms which are the companies or manufacturers in charge of removing recalled products from the market. Unsurprisingly, 95.5% of FDA recalls come from US-based recalling firms.
Canada has the most recalling firms of any foreign country, followed by Germany and the UK. Here’s a look at the top five foreign recalling firm countries in the FDA’s database:
- Canada: 891 products recalled
- Germany: 410 products recalled
- United Kingdom: 279 products recalled
- China: 277 products recalled
- Switzerland: 276 products recalled
In these cases, the products may be imported by US retailers, sold online, or distributed directly from other countries. The recalling firm is always responsible for letting their downstream accounts of the recall whether that’s consumers, retailers, or distributors. For any manufacturer, following a standard recall process is essential for compliance with the FDA’s requirements.
FDA recall centers
There are six centers that oversee FDA recalls, one for each product category. They are:
- Devices: Center for Devices and Radiological Health (CDRH)
- Food/Cosmetics: Human Foods Program (HFP)
- Biologics: Center for Biologics Evaluation and Research (CBER)
- Drugs: Center for Drug Evaluation and Research (CDER)
- Veterinary: Center for Veterinary Medicine (CVM)
- Tobacco: Center for Tobacco Products (CTP)
Recall information from each center is available on the FDA’s website for public access.
NOTE: The Human Foods Program replaced the Center for Food Safety and Applied Nutrition (CFSAN) in October 2024. Since we analyzed product recall data from 2012 to 2024, the dataset used the old CFSAN title to tag food/cosmetic recall events.
Recall classifications
The FDA organizes recalls into three different categories: Class I, Class II, or Class III. These classes are based on the potential health hazard severity of the recalled product.
Class I: Anyone who uses or is exposed to the product has a “reasonable probability” of suffering “serious adverse health consequences or death.”
Class II: Anyone who uses or is exposed to the product might “cause temporary or medically reversible adverse health consequences” or there’s only a “remote possibility” of serious consequences.
Class III: Anyone who uses or is exposed to the product is unlikely to suffer “adverse health consequences.”
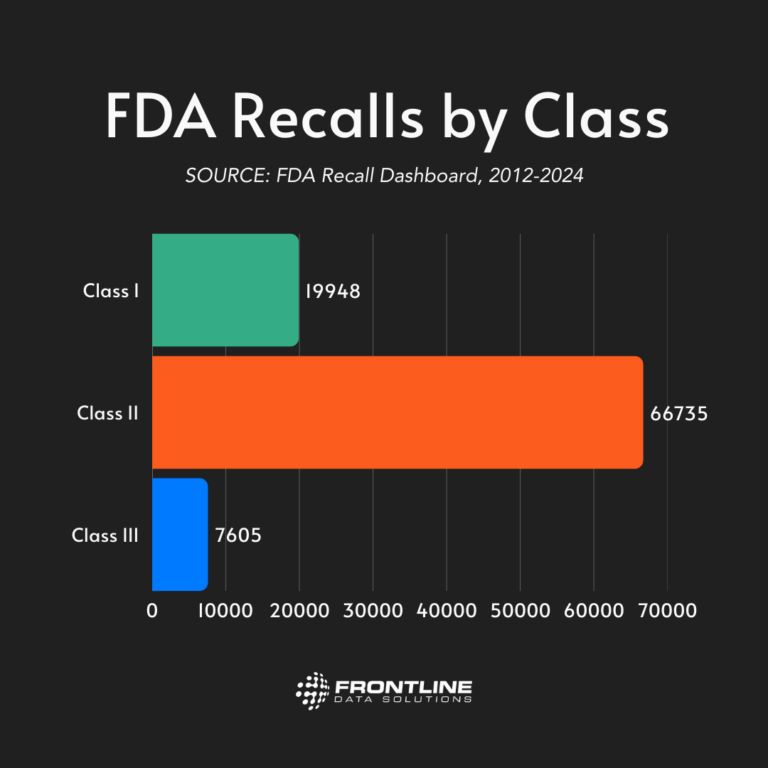
To better understand the classification system, we’ve pulled some examples from each class.
Class I recall examples
Health risks increase significantly for consumable products. That’s why it’s no surprise that 63.9% of all the food and cosmetics recalled were labeled as a Class I event. This includes many fresh vegetables like carrots, lettuce, and spinach which have the risk of E. coli contamination. Another common Class I food recall is meat products with potential salmonella risks. In these cases, the FDA doesn’t want to take any chances with a public outbreak of foodborne illness.
One lesser-known type of Class I recall is for software bugs in critical systems, especially in the medical field. For example, the FDA recalled a glucose ketone meter system because “the meter firmware [led] to transmission of erroneous glucose and/or ketone patient test results.” If doctors or patients use inaccurate test results to make health decisions, the consequences can be severe. Most people wouldn’t think of software as a danger to human health. But when you evaluate the impact of the issue, it’s clear to see why the FDA recalls these defective products.
Here’s a list of Class I recall examples from the FDA product recall data:
- Potential or confirmed contamination (e.g., E. Coli, salmonella, Listeria, etc.)
- Broken, detached, defective mechanical part or component
- Undeclared allergens in the product
- Critical malfunction of the product that results in potentially harmful outcomes
Class II recall examples
More than half of FDA recalls are Class II. So, it’s no surprise that this category includes all types of products.
After comparing Class I recalls to Class II recalls, there’s no clear delineation between the types of products recalled. In the Class II category, we see many similar products like supplements, blood products, tobacco snuff, fresh produce, and more.
The biggest difference between these categories is the reason behind the recall. For example, on December 6th, 2024, the FDA recalled L & L Homemade Corn Salsa because the salsa had a pH level of 4.7. The hazard was less severe because the FDA only detected a potential issue with the item. If they had found evidence of foodborne illness, the recall would have been classified as Class I.
Here are some examples of reasons for Class II events:
- Products deviated from CGMP regulations
- Non-sterile products exposed to microbial contaminants
- Foreign materials inside the product (shards of metal, plastic, etc.)
- Products skipped a quality control test
- Cross-contamination may have happened during production
Class III recall examples
Less than 10% of FDA recalls fall under the Class III category. That’s because these events carry a much smaller risk to public health. Regardless of risk, however, these recalls happen because the FDA deems it safer for the product to be off the market.
Here are some reasons for Class III recalls that happened between 2012 and 2024:
- Product wasn’t stored at an acceptable temperature during transport
- Expired ingredients used during manufacturing processes
- Blood or organs harvested from an ineligible donor
- Product causing false positive test results
- Improper labeling or branding of the products
Recalls by the year
Between 2012 and 2024, the total number of FDA recalls remained relatively stable until the COVID-19 pandemic. Upon analysis, the period between 2015 and 2017 had the most product recalls by far. In fact, the annual amount peaked in 2017 with 9,198 recalls.
The number has fallen steadily in subsequent years, hitting 5,309 in 2021. The COVID-19 pandemic played a big role in this drop, however. Since the low point, the number of annual recalls has begun increasing again, with more than 6,000 recalls in 2024.
It will be interesting to continue tracking annual product recall data and see if it regresses to pre-pandemic levels. Certainly, public awareness of FDA recalls has increased alongside the rise of social media. With more eyes on consumer safety, it’s possible that the FDA may issue more recalls in coming years to appease public sentiment.